Medical & Pharma
Jynneos: A Prevention for Smallpox and Monkeypox in Adults
The contagious disease
Monkeypox is a rare viral disease transmitted from animals to humans. This disease is caused due to an infection from monkeypox virus called variola virus, a member of the Orthopoxvirus genus, which causes symptoms similar to, however milder than, smallpox.

Smallpox was caused by the variola virus which was transmitted from person to person through direct contact via contaminated mediums such as air, fluid, etc. By 1980, smallpox was eradicated worldwide after a global vaccination campaign. Therefore this deadly contagious disease no longer occurs naturally.
Jynneos: A prevention
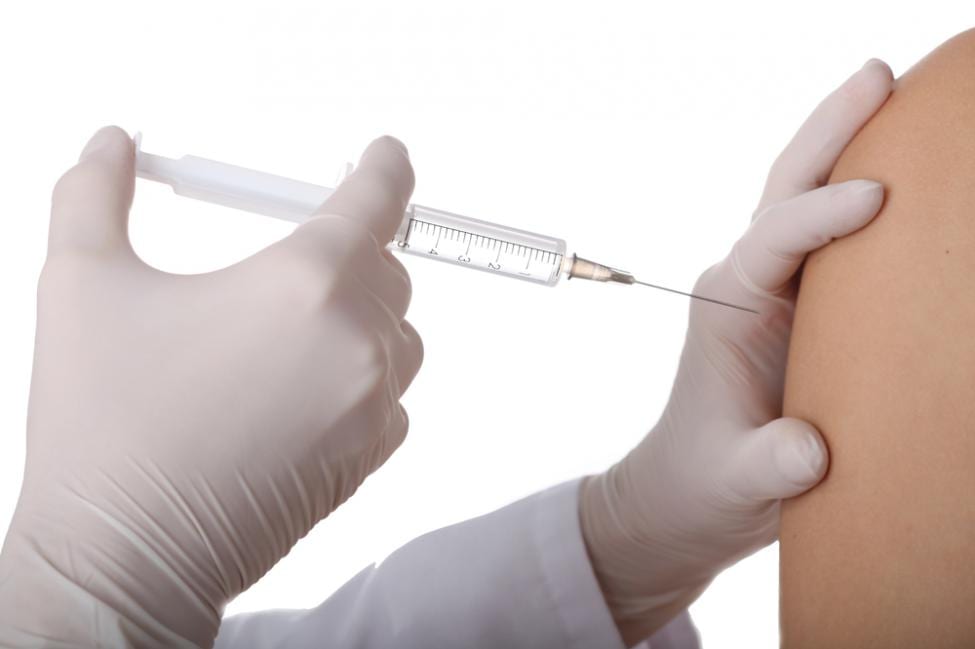
Recently, the Food and Drug Administration of the United States has approved the Jynneos Smallpox and Monkeypox Vaccine, for the prevention of smallpox and monkeypox diseases in adults or human beings over 18 years of age. This is the only currently FDA-approved vaccination for the prevention of monkeypox disease.
According to Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, “Following the global Smallpox Eradication Program, the World Health Organization certified the eradication of naturally occurring smallpox disease in 1980. Routine vaccination of The American public was stopped in 1972 after the disease was eradicated in the US and, as a result, a large proportion of the U.S., as well as the global population has no immunity.”
Further, he added, “Therefore, although naturally occurring smallpox disease is no longer a global threat, the intentional release of this highly contagious virus could have a devastating effect. Today’s approval reflects the U.S. government’s commitment to preparedness through support for the development of safe and effective vaccines, therapeutics, and other medical countermeasures.”
Jynneos: The Vaccine
Jynneos is a suspension for the subcutaneous vaccine based on a live, modified Vaccinia Ankara, MVA-BN, which is incompetent of replicating in the body, however at the same time capable of eliciting a potent immune response. Jynneos vaccine was developed by Bavarian Nordic A/S in partnership with the U.S. Government to prevent people from smallpox. It also prevents the people with weak immune systems or who are at high risk or had adverse reactions to previous smallpox vaccines and is based on replicating vaccinia virus strains.
Some of the Side Effects
The most common adverse effects associated with Jynneos include pain, redness, swelling, induration, and itching. Similarly, systemic adverse reactions include muscle pain, fatigue, headache, nausea, myalgia, and chills. Serious adverse effects include Crohn’s disease, sarcoidosis, extraocular muscle paresis, and throat tightness. However, only 0.05% of the subjects reported such serious adverse reactions. Further, Cardiac adverse impacts of special interest were reported in 0.08% of the subjects who received Jynneos and included tachycardia, electrocardiogram T wave inversion, electrocardiogram abnormal, electrocardiogram ST-segment elevation, electrocardiogram T wave abnormal, and palpitations.
Thus we can conclude that side effects are very rare and we have a safe medicine for Monkeypox and Smallpox.