Delhi
Lockdown Update: Some services to resume

Recent Highlights of lockdown and coronavirus:
- Partial upliftment of lockdown in the state capital by Delhi Government.
- Only essential services allowed, nationwide lockdown to act till May 3.
- ICMR requests faulty kits to be shipped back to China, suggests halting further procurement.
- Vaccine candidates enter trials worldwide.
The Delhi government has decided to partially relax the continuing lockdown. It decided to open some amenities in the capital. Delhi has seen more than 3,000 coronavirus cases to date.

The Kejriwal government has allowed a few services to start functioning from 28th April. These include pathological laboratories, book stores, and plumbing services.
Delhi Disaster Management Authority (DDMA) has also permitted inter-state travel for health workers, lab technicians, and scientists.
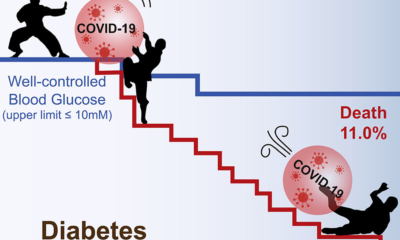
The number of containment zones in Delhi is now at 99. There have been 54 deaths reported with over 3,100 coronavirus cases. The Delhi government still announced the easing of the lockdown. Though it is partial and the lockdown will continue till May 3 till further correspondence is made.
A list of services being opened up amid the partial easing are:
- Inter and intrastate movements by all healthcare workers and scientists have been permitted, including by air if necessary.
- Healthcare: Dispensaries, clinics and pathological laboratories, sale, and supply of vaccine and medicine, and Vet hospitals.
- Shelter homes for children, disabled, mentally challenged, senior citizens, destitute, women, and widows.
- Self-employed persons such as electricians, plumbers or those repairing water purifiers can start providing their services
- Electric fan shops have been allowed to open.
- School books shops selling educational books are also allowed to open.
The nationwide lockdown that started on March 25 was extended till May 3 as the cases rose to an all-time high. MHA issued a set of thorough guidelines for all states and Union Territories to follow a day after.
The Delhi government has declared some relaxations after scrutinizing the COVID-19 situation in the state.
Meanwhile, the Indian Council For Medical Research has urged the state governments to halt the further acquisition of rapid antibody test kits. The results were not found satisfactory and many kits were deemed faulty. China has been shipping these kits to India. But so far the results of these kits have been dismal. ICMR suggested that these faulty kits be shipped back to China.
There has also been news that human trials have begun for coronavirus vaccine. There are at least seven vaccine candidates that have entered into trials across the world.
Such developments take many experiments and go through various stages. Usually, developing a cure might take up to a decade. But seeing the merciless onslaught of the COVID-19 virus, scientists are forced into taking shortcuts to develop the vaccines. Yet, the timeline is set at 1.5 years, according to experts.
In India, firms are engrossed in pre-clinical research for vaccine development. Ahmedabad based Pharma major Zydus Cadila along with Hyderabad-based biopharma firm Biological E Limited and Pune-based vaccine manufacturer Serum Institute of India are leading the research.
Stay home, stay safe, and follow the protocols to protect yourself and your loved ones.